11.11.2020
Experiment Leaders: Brady Bresnahan and Supriya Ghosh
On November 9th and 11th, 2020, the Murray Middle School Astronomy class hosted by Mrs. Michelle Ross explored the relationship between rust, rotting, and rocket research in a virtual setting with Science for All. Students were able to build on their knowledge of the rusting of metals and the rotting of food that they had experienced first hand in their everyday lives, and apply that knowledge to the design and testing of aerospace materials. The lesson goals were to teach students about oxidation reactions of metals, food, and aerospace materials and to demonstrate how scientists controlled and tested these reactions.
After introductions of the event leaders and volunteers, the students were first shown live examples of freshly cut fruits - an apple and an avocado, with the goal to see what would happen to them during the end of the visit when left outside. Following this, the students had a chance to learn about rusting of metals in the ambient atmosphere via a demonstration of a time-lapse of a piece of iron, when placed outside. These were used to explain the oxidation reaction in these metals and the components necessary for them. The discussion then moved on to rotting of food items, which is an identical chemical reaction involving the degradation of the chemicals and enzymes in the food by microorganisms.
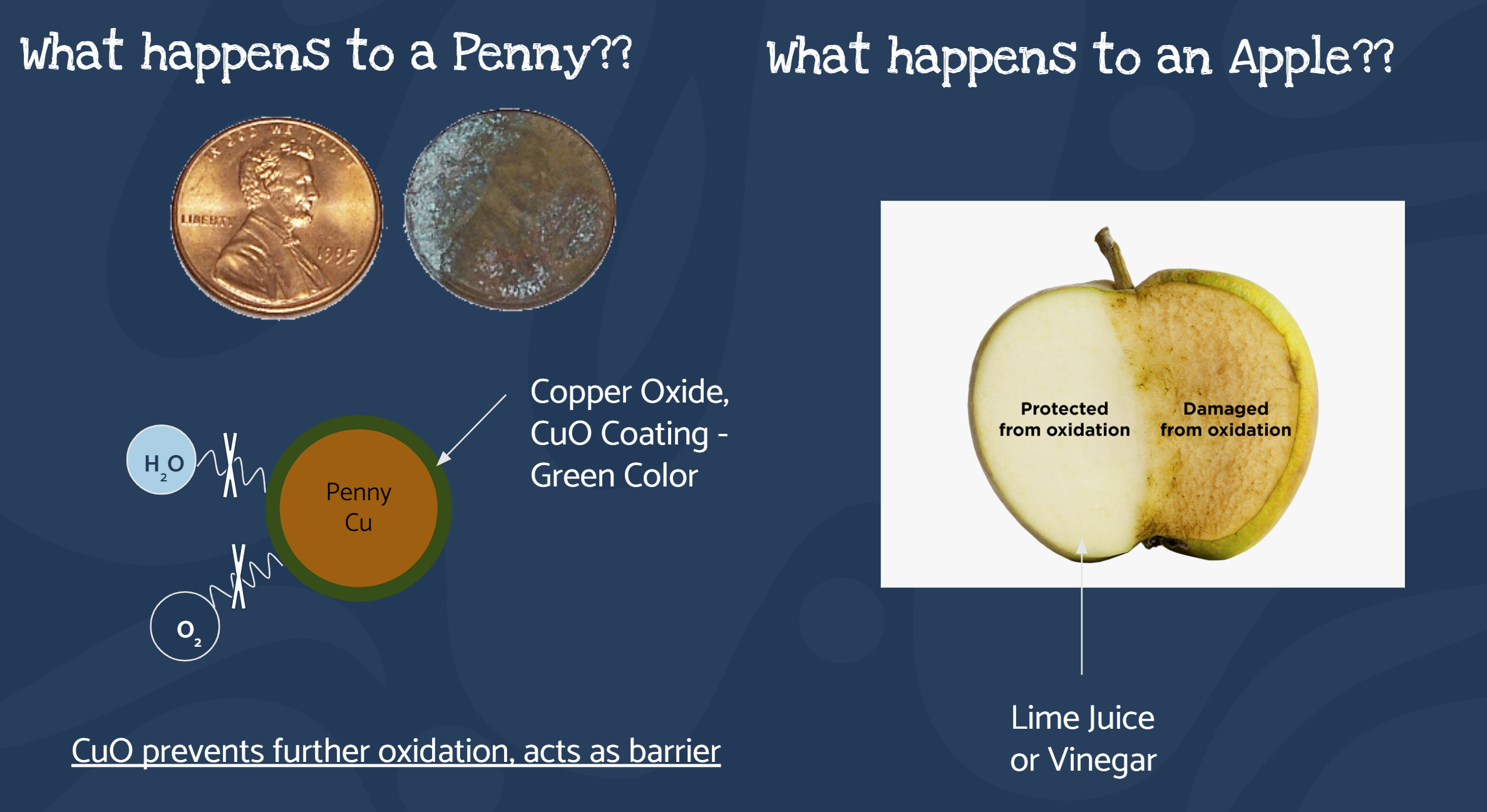
Further, protective strategies against oxidation reactions in both systems were discussed through examples, where the students were asked to think about “Why a penny doesn’t rust and completely degrade?” and “What has happened to the apple and avocado we cut earlier in the class”. This allowed the students to talk about the role of protective coatings that can act as barriers to oxygen and moisture preventing rusting. Further, roles of storing food products in tupperwares and cool temperatures were discussed.
To demonstrate how scientists and engineers control and test these oxidative reactions on aerospace materials, Brady gave a guided tour of the Poerschke Group laboratory, at the Department of Chemical Engineering and Materials Science, UMN. The tour started with the fabrication of materials using a spark plasma sintering instrument that turned metal powders into dense solids by using electrical heat and pressure. The body of the tour focused on the differences between tube and box furnaces that both work like ovens and heat samples. Tube furnaces allow for the gas around the sample to be controlled and allow the user to control the oxidation reaction. Box furnaces, on the other hand, don’t have control over the gas around the sample but have larger volumes for samples. To end the tour, Brady showed how glass blowing can be used to seal samples in tupperware-like tubes that would prevent oxygen from oxidizing the sample. Throughout the tour, safety equipment was shown and highlighted to show students the importance of safety in a laboratory.

After the tour, students broke into groups to have discussions with SFA volunteers about how to prevent rust and rotting, and aspects of the lab tour. Although the virtual setting wasn’t ideal, the students seemed to enjoy the special lesson and showed great improvement from the pre and post quizzes. Before the session ended, we did revisit the apple and avocado from the beginning of the class to see how even a small time outside was sufficient to oxidize them!